

iSTAR Medical is committed to delivering breakthrough eye care solutions to patients through minimally invasive implants.

MINIject® is iSTAR Medical’s most advanced product, and is approved in Europe for the treatment of Glaucoma – the leading cause of irreversible blindness.1 Minimally-Invasive Glaucoma Surgery (MIGS) devices are considered the future in glaucoma treatment.2 In clinical trials, MINIject has been shown to provide safe, meaningful and sustained treatment to lower eye pressure for patients with primary, open-angle glaucoma.3
LEARN MORE ABOUT MINIJECT
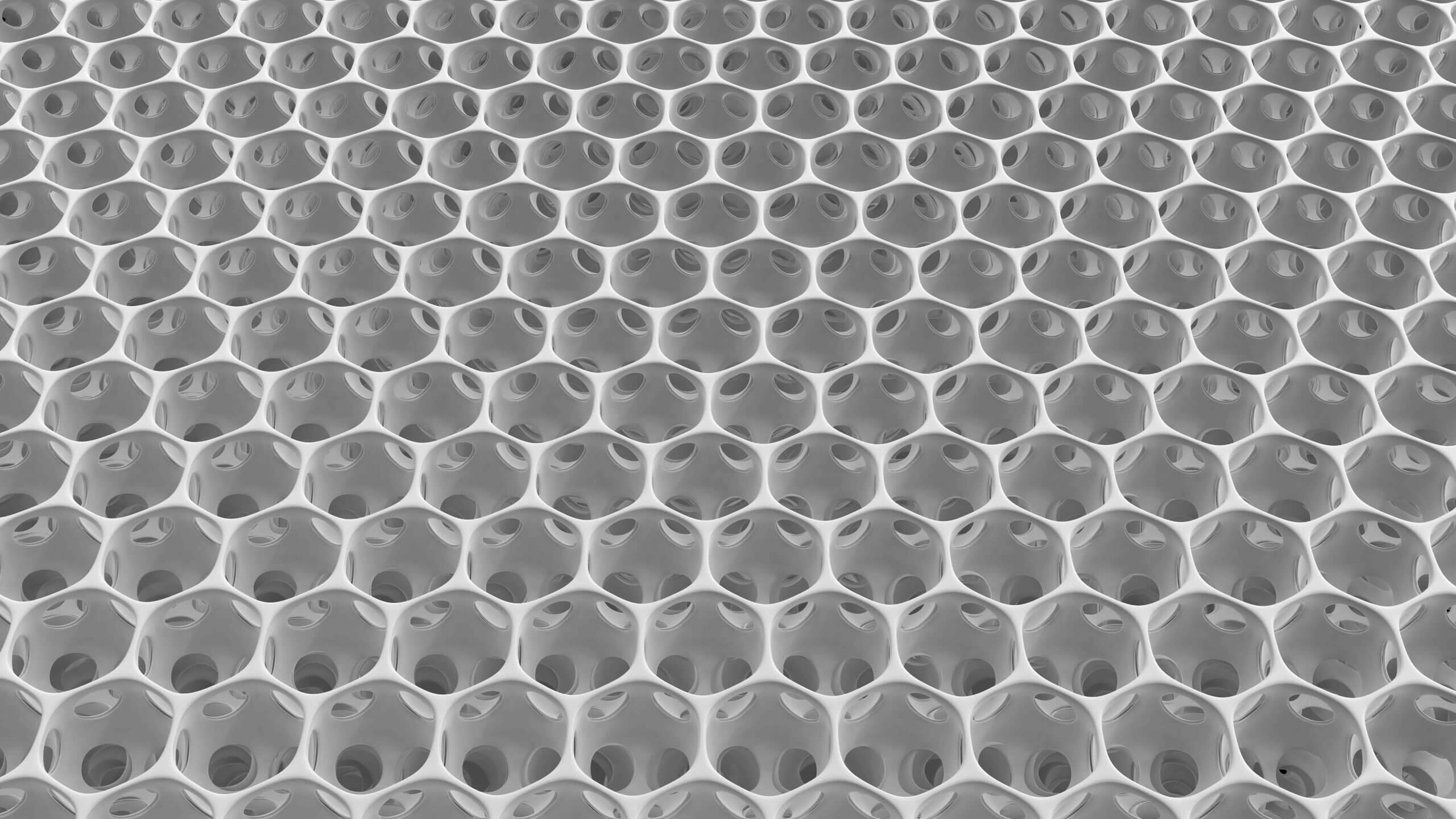
MINIject®’s distinctive tissue-integrating capabilities unlock a safe and effective option for patients. Its material, STAR®, has outstanding anti-fibrotic and anti-inflammatory properties due to its unique porous structure made of medical-grade silicone, and was developed by the University of Washington, Seattle (USA).4
iSTAR Medical’s management team and board have experienced end-to-end product development, clinical, regulatory and market access capabilities. iSTAR Medical is aiming to build an exceptional team and pipeline of high quality products like MINIject® seeking to establish new treatment paradigms in eye care conditions with the highest patient needs.
 |
 |
 |
 |
 |
 |
 |
 |
Investors listed are those with a shareholding greater than 3%.
iSTAR Medical is ISO 13485 , MDR and MDSAP Australia and Canada BSI certified.
MINIject® is only available for sale in the European Union, the UK, Norway, Iceland, Switzerland and Australia.
1 Jonas JB, Aung T, Bourne RR et al. “Glaucoma”. Lancet 2017; 390: 2083–93
2 Market Scope, “2021 Glaucoma Surgical Device Market Report”, July 2021
3 Denis P, Hirneiß C, Durr GM, et al. “Two-year outcomes of the MINIject drainage system for uncontrolled glaucoma from the STAR-I first-in-human trial”. Br J Ophthalmol 2020;0:1–6
4 Grierson I, Minckler D, Rippy MK et al. “A novel suprachoroidal microinvasive glaucoma implant: in vivo biocompatibility and biointegration.” BMC biomed eng 2, 10 (2020).